Precision Stamping Die Components in Medical Devices
Keywords: precision stamping, die components, medical device manufacturing, metal stamping tools, micro stamping, medical-grade dies, high-precision tooling
Introduction: Why Precision Tooling Matters in Medical Device Manufacturing
In the medical device industry, precision is not optional—it is essential. From implantable components to surgical instruments and disposable devices, every part must meet strict standards for accuracy, hygiene, and repeatability.
At the heart of many of these components is precision metal stamping, made possible by high-performance stamping dies and their components. This article explains the key stamping die components used in medical applications and how they enable the production of safe, reliable, and cost-effective medical products.
Core Stamping Die Components Used in Precision Manufacturing
Stamping dies are complex tools made up of multiple engineered components. In high-end sectors like medical manufacturing, each component must deliver consistent performance over millions of cycles.
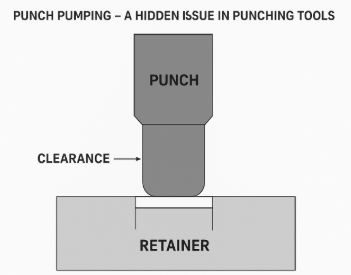
1. Punches and Dies (Forming Components)
- Responsible for cutting, bending, or forming sheet metal into the required part geometry
- Require ultra-tight tolerances (±2–5 microns)
- Materials used: tungsten carbide, powder high-speed steel (such as ASP60), or hardened stainless steel
2. Guide Posts and Guide Bushings
- Maintain alignment between upper and lower die plates
- Critical for high-precision applications like micro stamping of tiny components
- Reduce wear and improve tool longevity
3. Strippers and Ejectors
- Remove the stamped part or scrap from the tooling after forming
- May include spring strippers, nitrogen cylinders, and ejector pins
- Enhance cycle time and reduce part deformation
4. Locating and Positioning Elements
- Includes dowel pins, locating blocks, and material stops
- Ensure accurate placement of the metal sheet or strip
- Essential for consistent part production, especially in multi-cavity dies
5. Die Sets and Plates
- Provide the structural support for the stamping tool
- Must resist deformation under high loads and automated production environments
- Often custom-designed for cleanroom compatibility and modular integration
Medical Applications That Rely on Precision Die Components
The following are examples of medical parts manufactured using precision stamping, along with the critical die components involved:
| Medical Component | Relevant Die Components |
|---|---|
| Surgical instrument housings | High-precision punches, die inserts, guide assemblies |
| Disposable clips and brackets | Multi-stage dies, strippers, ejectors |
| Catheter connectors | Locating pins, guide plates, ejector systems |
| Implantable stent frames | Micro-stamping dies, carbide punches, high-tolerance bushings |
| Respiratory device frames | Mold bases, die shoes, alignment systems |
Requirements for Stamping Dies in the Medical Industry
Medical manufacturing imposes some of the strictest requirements on stamping tools and components. Key specifications include:
| Requirement | Description |
|---|---|
| Material standards | Biocompatible stainless steels (304, 316L), wear-resistant coatings (TiCN, DLC) |
| Surface finish | Mirror-polished, burr-free, scratch-resistant surfaces for hygienic applications |
| Dimensional tolerance | ±2 to ±5 microns for mating and interlocking parts |
| Tool longevity | 1 million+ cycles per tool for cost-efficiency and reliability |
| Compliance | Tooling must support ISO 13485, FDA, and CE manufacturing standards |
Industry Trends in 2025 and Beyond
- Micro-stamping is driving demand for ultra-precision tools for smaller and thinner components
- Modular tooling systems are enabling faster changeovers and reduced downtime
- In-die monitoring (sensors and counters) is improving quality control
- Sustainability and material recyclability are increasingly important in tooling design
Conclusion
Precision stamping die components are the foundation of scalable and high-quality medical device manufacturing. They ensure the repeatability, dimensional accuracy, and surface integrity required by regulatory agencies and patient safety standards.